Infrared spectroscopy
The absorption spectrum of molecules and solids contains a wealth of information regarding their properties on a microscopic scale. While the spectrum in the ultraviolet and visible spectral range (UVvis) reflects electronic properties, the absorption signature in the infrared (IR) provides access to vibrational properties.
The IR spectrum consists of absorption bands associated with specific molecular vibrational modes and thus reflects chemical structure and structural changes. Electronic excitation can induce such changes – as, for instance in a photoswitchable molecule or in the structural rearrangement around an added charge. Therefore, these processes can be observed with the help of IR spectroscopy.
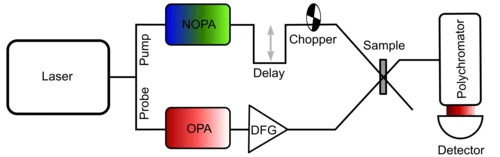
Vibrational relaxation and structural changes at the molecular level occur on a time scale of femtoseconds to picoseconds (10-15-10-12 s), which can be accessed via pump-probe spectroscopy. Here, electronic or vibrational excitation of the sample is achieved by a short laser pulse. A second pulse, which can be delayed in time, allows to probe the induced absorption changes in the excited state.
UVvis-pump IR-probe spectroscopy
Charge carrier dynamics in polymers and perovskites
Solar cells based on perovskites or conjugated polymers promise high efficiencies combined with ease of processing and cost effectiveness. Generation, diffusion and recombination processes of charge carriers in these materials, such as excitons and polarons, can be identified and investigated on time scales of femtoseconds to picoseconds. In the IR spectral range, broad electronic signals, like absorption due to free charge carriers or polarons, as well as sharp absorption signatures associated with specific molecular vibrational modes can be observed. Such infrared-activated vibrations (IRAVs), which occur in the presence of a charge, can serve as sensitive indicator modes for the dynamics of different charge carrier species.
Publications:
- K. Stallhofer, M. Nuber, R. Kienberger, V. Körstgens, P. Müller-Buschbaum & H. Iglev: "Dynamics of Short-Lived Polaron Pairs and Polarons in Polythiophene Derivatives Observed via Infrared-Activated Vibrations", Journal of Physical Chemistry C, 123, 46, 28100-28105
- K. Stallhofer, M. Nuber, D. Cortecchia, A. Bruno, R. Kienberger, F. Deschler, C. Soci & H. Iglev: "Picosecond Charge Localization Dynamics in CH3NH3PbI3 Perovskite Probed by Infrared-Activated Vibrations", The Journal of Physical Chemistry Letters 2021 12 (18), 4428-4433
- M. Nuber, D. Sandner, T. Neumann, R. Kienberger, F. Deschler & H. Iglev: "Bimolecular Generation of Excitonic Luminescence from Dark Photoexcitations in Ruddlesden–Popper Hybrid Metal-Halide Perovskites", The Journal of Physical Chemistry Letters 2021 12 (42), 10450-10456
- M. Nuber, L. V. Spanier, S. Roth, G. N. Vayssilov, R. Kienberger, P. Müller-Buschbaum & H. Iglev: "Picosecond Charge-Transfer-State Dynamics in Wide Band Gap Polymer–Non-Fullerene Small-Molecule Blend Films Investigated via Transient Infrared Spectroscopy", J. Phys. Chem. Lett. 2022, 13, 44, 10418–10423
- M. Nuber, Q. Y. Tang, D. Sandner, J. Yin, R. Kienberger, C. Soci & H. Iglev : "Accelerated polaron formation in perovskite quantum dots monitored via picosecond infrared spectroscopy", J. Mater. Chem. C, 2023,11, 3581-3587
Vibrational relaxation and molecular switching processes
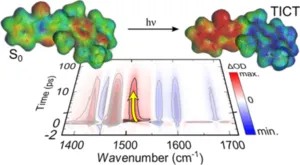
Vibrational relaxation and the direct observation of the evolution of structural changes are typical tasks of molecular spectroscopy. An example is the investigation of light-induced molecular switching processes on a picosecond time scale or charge transfer during photocatalysis.
Publications:
- K. Stallhofer, M. Nuber, F. Schüppel, S. Thumser, H. Iglev, R. de Vivie-Riedle, W. Zinth, H. Dube: "Electronic and Geometric Characterization of TICT Formation in Hemithioindigo Photoswitches by Picosecond Infrared Spectroscopy" J. Phys. Chem. A</em> 2021, 125, 20, 4390–4400
IR-pump IR-probe spectroscopy on ice and hydrate models
The macroscopic properties of water and ice are determined by the underlying network of hydrogen bonds, which is characterized by a multitude of different bonding motives. Salt hydrates, which feature water molecules or small water clusters incorporated into their crystal structure, constitute well-defined model systems to study the microscopic structure of water and its dynamics.
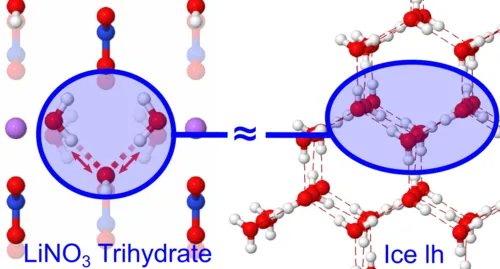
Publications:
- D. Hutzler, K. Stallhofer, R. Kienberger, E. Riedle & H. Iglev: "Icelike Vibrational Properties of Strong Hydrogen Bonds in Hydrated Lithium Nitrate" J. Phys. Chem. A 2020, 124, 28, 5784–5789 (2020)
- D. Hutzler, Ch. Brunner, P. Petkov, T. Heine, S. Fischer, E. Riedle, R. Kienberger & H. Iglev:„Dynamics of the OH stretching mode in crystalline Ba(ClO4)2·3H2O “, J. Chem. Phys. 148, 054307 (2018)
- “Highly Selective Relaxation of the OH Stretching Overtones in Isolated HDO Molecules Observed by Infrared Pump-Repump-Probe Spectroscopy”, Journal of Physical Chemistry 119, Issue 26, 6831-6836 (2015)
- "A novel setup for femtosecond pump-repump-probe IR spectroscopy with few cycle CEP stable pulses," Opt. Express 21, 20145-20158 (2013)
- “Dynamics of weak, bifurcated and strong hydrogen bonds in lithium nitrate trihydrate”, J. Phys. Chem. Lett. 2, 1633–1638 (2011)