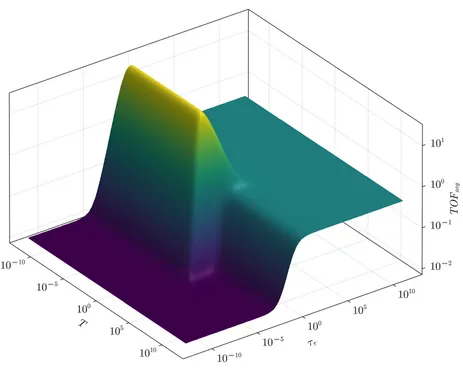
Dynamic catalysis aims to overcome the limitations of conventional catalysts by periodically changing the surface binding energy of intermediates on the catalyst surface. The oscillation parameters can be adjusted to create optimum conditions for each reaction step. In this way, Sabatier's principle, according to which the optimum catalyst should not bind too strongly or too weakly to the reaction intermediate, can be circumvented, leading to a considerable increase in the overall reaction rate and product selectivity. Dynamic catalysis is studied on a theoretical level using nonlinear differential equations for the reaction rate, which can be adapted to very general reaction rate schemes, but also to specific reactions. We apply dynamic catalysis in particular to electrocatalytic reactions such as hydrogen evolution or carbon dioxide reduction, concentrating initially on theoretical predictions that will be validated experimentally in a second step.